Life Sciences Industry
Measure Your Program Outcomes

- 90%
reduction in the time taken to manage compliance activities
- 80%
improvement in
risk and control
framework-related operational efficiency
- 67%
improvement in risk reporting visibility and efficiency for the executive management and board

Boost Risk and Compliance Visibility and Ensure Better Outcomes
Pharmaceutical, medical devices, and clinical research companies face an increasing amount of regulatory scrutiny and risks. Over the last few years, organizations have paid substantial fines for non-compliance. Regulators worldwide have started multiple initiatives to get lower-cost drugs and devices to market and accelerate the R&D process. Organizations are transforming their approach to research, clinical trials, manufacturing, supply chain, patient engagements, and promotions which impacted their overall risk profile. MetricStream helps life sciences companies adopt an integrated approach to GRC to effectively manage risk, compliance, audit, cyber risks, third-party, and business continuity programs, enabling resilience and assurance to management, the board, and regulators.
How MetricStream Software Solutions Help You

Implement an Integrated Approach to Risk Management
Efficiently manage and mitigate multiple types of risk, including enterprise risk, compliance risks, quality risks, device risks, marketing risks, and others. With MetricStream Enterprise Risk Management, establish a comprehensive risk strategy that automates risk identification, assessments, monitoring, and acceptance across all the various business units and functions as required. Improve risk visibility and foresight, gain real-time insights, and prioritize investments and actions based on the quantified impact of risks.

Keep Up with Evolving Regulatory Compliance Obligations
Easily navigate the complex web of regulatory requirements governing life sciences organizations. Strengthen compliance by effectively tracking and managing regulatory changes with MetricStream Regulatory Compliance. Adopt an integrated approach to stay on top of changing regulatory obligations, simplify and streamline the management of policies, perform compliance assessments, and manage cases and regulatory audits. Reduce the cost of compliance and eliminate process inefficiencies and redundancies.

Gain Visibility into Third-Party Risk Exposures
Gain an integrated, real-time view of risks across all third parties, including vendors, suppliers, and contractors. Monitor and manage the risks associated with extended enterprise alongside other risks and protect your business from existing and potential threats from third and related fourth parties. MetricStream Third-Party Risk Management simplifies managing risk and compliance across the third-party lifecycle, including onboarding, due diligence, continuous monitoring, performance management, and offboarding.

Strengthen Cyber Resilience
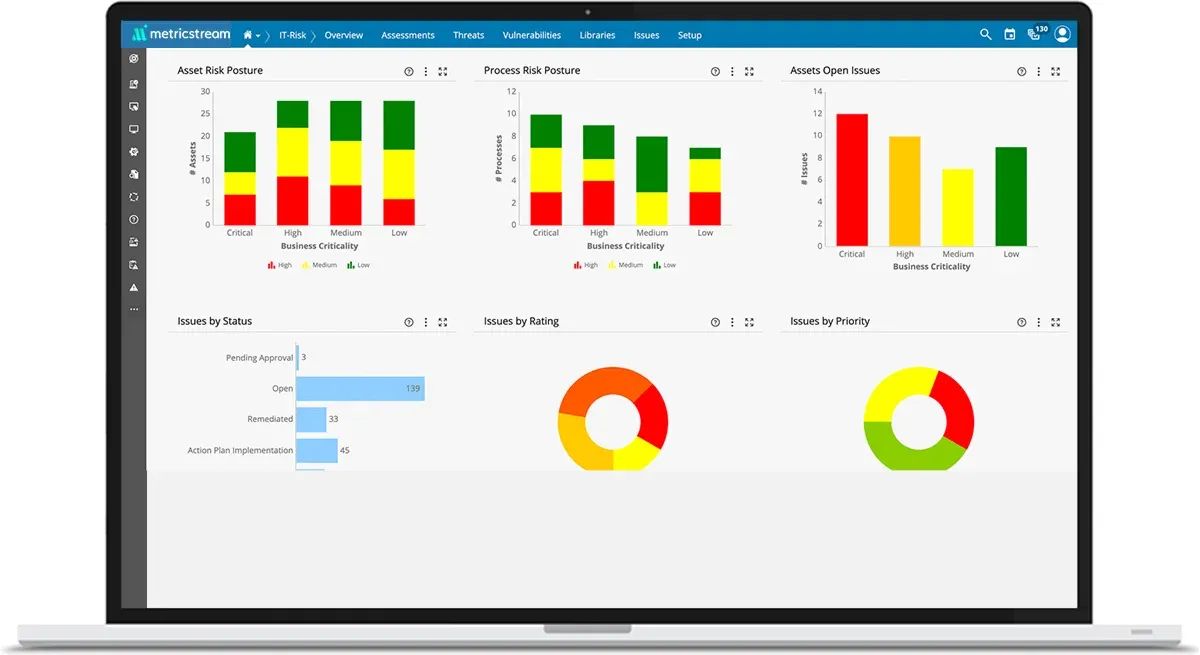
Gain a 360-degree, real-time view of IT and cyber risk, threats, vulnerabilities, and associated controls across the organization. Simplify the identification and analysis of multiple risks in IT operations and contextualize IT risks based on the associated processes, business units, and IT assets. With MetricStream CyberGRC, ensure alignment in strategy and minimize potential issues while adhering to compliance regulations and frameworks such as ISO 27001, NIST CSF, NIST SP800-53, and others all in one place.
How MetricStream Benefits Your Business
- Strong risk-based decision-making and governance across the entire enterprise with accurate and timely risk insights
- Reduce compliance violations and fines, improve confidence with regulators and executive management through a robust, integrated approach to risk, resilience, and compliance
- Gain real-time visibility into IT and cyber risks, threats, and vulnerabilities, and prioritize risk mitigation measures and investments
- Proactively manage third-party, supplier, and vendor risks by automating onboarding, due diligence, continuous monitoring, and more